 Introduction
Introduction
Strangles is a highly contagious and serious infection of horses and other equids caused by the bacterium, Streptococcus equi. The disease is characterized by severe inflammation of the mucosa of the head and throat, with extensive swelling and often rupture of the lymph nodes, which produces large amounts of thick, creamy pus.
Strangles is caused by Streptococcus equi subspecies equi, better known as Streptococcus equi (S. equi). The organism can be isolated from the nose or lymph nodes of affected animals, and is usually readily identified in the laboratory by simple sugar tests.
Transmission and Environmental Survival
Horses of all ages are susceptible, though strangles is most common in animals less than 5 years of age and especially in groups of weanling foals or yearlings. Foals under 4 months of age are usually protected by colostrum-derived passive immunity. (1) S. equi is main-tained in the horse population by carrier horses but does not survive for more than 6–8 weeks in the environment. Although the organism is not very robust, the infection is highly contagious. Transmission is either by direct or indirect contact of susceptible animals with a diseased horse. Direct contact includes contact with a horse that is incubating strangles or has just recovered from the infection, or with an apparently clinically unaffected long-term carrier. Indirect contact occurs when an animal comes in contact with a contaminated stable (buckets, feed, walls, doors) or pasture environment (grass, fences, but almost always the water troughs), or through flies. (2)
Clinical Illness
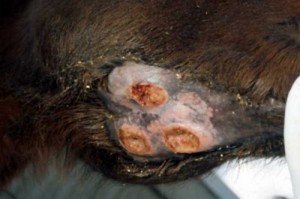
Susceptible horses develop strangles within 3–14 days of exposure. (2) Animals show typical signs of a generalized infectious process (depression, inappetence, and fever of 39°C–39.5°C). More typically of strangles, horses develop a nasal discharge (initially mucoid, rapidly thickening and purulent), a soft cough and slight but painful swelling between the mandibles, with swelling of the submandibular lymph node. Horses are often seen positioning their heads low and extended, so as to relieve the throat and lymph node pain.
With the progression of the disease, abscesses develop in the submandibular (between the jaw bones) and/or retropharyngeal (at the back of the throat) lymph nodes. The lymph nodes become hard and very painful, and may obstruct breathing (“strangles”). The lymph node abscesses will burst (or can be lanced) in 7–14 days, releasing thick pus heavily contaminated with S. equi. The horse will usually rapidly recover once abscesses have ruptured.
Although the disease process described above is classic, some horses (especially older animals) will develop a mild, short lasting disease without or with minor lymph node abscessation. This is thought to be the result of partial immunity although this may also result from infection by S. equi of relatively low virulence. Classic strangles is a severe infection that can be fatal, usually because of a variety of complications that occur.
The main and often fatal complications of strangles are:
Bastard strangles, which describes the dissemination of infection to unusual sites other than the lymph nodes draining the throat. For example, abdominal or lung lymph nodes may develop abscesses and rupture, sometimes weeks or longer after the infection seems to have resolved. A brain abscess may rupture causing sudden death or a retropharyngeal lymph node abscess may burst in the throat and the pus will be inhaled into the lung.
Purpura haemorrhagica, which is an immune-mediated acute inflammation of peripheral blood vessels that occurs within 4 weeks of strangles, while the animal is convalescing. It results from the formation of immune complexes between the horse’s antibodies and bacterial components. These immune complexes become trapped in capillaries where they cause inflammation, visible in the mucous membranes as pinpoint haemorrhages. These haemorrhages lead to a widespread severe edema of the head, limbs, and other parts of the body. Purpura can also be a complication of routine vaccination.
Minor, non-fatal complications include:
Post strangles myocarditis (inflammation of heart muscle), which may follow strangles in a small proportion of horses. An electrocardiogram (ECG) can determine that a horse can return to heavy work or to training after an episode of strangles.
Purulent cellulitis (inflammation of the subcutaneous tissue), which is an unusual occurrence where infection spreads locally in the subcutaneous tissue to the head.
Laryngeal hemiplegia, which involves paralysis of the throat muscles. It is commonly referred to as “roaring”. The condition may follow abscessation of cervical lymph nodes.
Anaemia (low red blood cell count), during the convalescent period because of immune-mediated lysis of red blood cells.
Guttural pouch empyaema (filled with pus), which may be concurrent with classic strangles, or follow in the immediate convalescent period. The 2 guttural pouches are large mucous sacs; each is a ventral diverticulum of the Eustachian tube. They are present only in Equidae and are situated between the base of the cranium dorsally and the pharynx ventrally. (3) They open into the nasal pharynx and each has a capacity of about 300 mL. (4) Persistent infection in the guttural pouch may lead to inspissation (drying) of pus and, in some cases, the formation of a solid, stone-like, concretion called a chondroid. Animals that have persistent infection of the guttural pouches become the carriers, the major source of infection to spark outbreaks in susceptible horses with which they are mixed.
Apart from the problem of long-term guttural pouch carriers, discussed below, recovered horses may shed S. equi from their nose and in their saliva for up to 6 weeks following infection. Therefore, isolate all horses that have had strangles from susceptible animals for 6 weeks following infection.
Diagnosis and Treatment
Diagnosis can be confirmed by culturing pus from the nose, from abscessated lymph nodes or from the throat of clinically affected horses. Although S. equi isolates are thought to be genetically identical, isolates may vary in virulence and atypical isolates occur, which differ in their sugar tests from typical S. equi.
There is argument among veterinarians as to whether or not to treat an animal with strangles with antibiotics. Many veterinarians think that treatment will impair the development of immunity and may predispose an animal to prolonged infection and to bastard strangles. Treatment of a horse in the early stages of strangles is usually effective and is not associated with untoward effects. The causative agent is highly susceptible to penicillin G. If the disease is more advanced, then most veterinarians will not use antibiotics but rather will recommend nursing care and trying to hasten the development of abscesses (which can be drained) by poulticing. Antibiotics may, however, be used if complications arise.
Prevention of Strangles
Detection of carriers
In recent years, work in the United Kingdom has added substantially to the understanding of the carrier state in strangles. (5) This work has shown that carriers are usually horses that, following recovery from clinical illness, remain with persistent infection of the guttural pouches. This infection is associated with persistent, purulent inflammation in this site or, in some cases, with the presence of chondroids. These carriers can be detected either by culture or by detection of S. equi DNA using the polymerase chain reaction (PCR) test. PCR is a more sensitive test but also is currently more expensive. The combination of these tests may be even more reliable, but is expensive.
Because the organism is adapted to the horse, a system of control based on detection, isolation and treatment of carriers could potentially be used to eradicate the organism on a continent-wide basis. Horse owners and veterinarians have not yet organized to take advantage of this new understanding. However, vaccination with a live vaccine may interfere with the detection and eradication approach to control.
A series of 3 nasopharyngeal swabs (e.g., swabs introduced through the nose and collecting material from the back of the throat), evenly spaced over 2 or 3 weeks, will result in the detection of about 60% of carriers using isolation and identification of the organism, or of about 90% of carriers using PCR. For the detection of carriers, the laboratory should use a selective medium (Columbia blood agar with nalidixic acid and colistin).
Investigation of carriers should be done either before a new animal is introduced into a stable or herd, or at least 30 days following recovery of a horse from strangles. Animals should be isolated until there have been 3 consecutive negative cultures and/or PCR reactions.
If an animal is positive, endoscopic evaluation of the guttural pouch is recommended, chondroids removed, and guttural pouches treated by flushing and infusion of 5 million units of penicillin G in 3% gelatin. In addition, these horses should be treated with penicillin G intramuscularly for 7 days, isolated for 30 days, and then retested with the 3 consecutive series of nasopharyngeal swabs and culture. PCR is not usually recommended in these animals because it is so sensitive that it may identify dead bacteria and so give a “positive” reaction. Animals that remain positive should go through a repeat treatment and culture cycle.
This system of identification of carriers by culture and/or PCR, while not 100% reliable, is more reliable than the usual recommendation for the control of strangles. These are to isolate or quarantine new arrivals for 2–3 weeks, look for evidence of strangles-like upper respiratory tract infection, and carry out one or more nasal swabs that are used for culture. Your veterinarian will be able to give you the current laboratory costs per test for bacteria isolation and for the PCR test. Owners may not be prepared to take this route to control strangles due to the financial costs.
Vaccination
Both a killed and a live vaccine are available for the control of strangles. The only killed vaccine currently available in Canada is StrepguardTM by Intervet. Killed vaccines, in general, are administered with an initial series of intramuscular injections followed by an annual booster. There may be adverse reactions at the injection site (marked pain, even frank abscesses). Some animals have even developed purpura haemorrhagica following vaccination. The killed vaccines do not provide complete protection because they do not result in the local, nasopharyngeal antibodies thought to be important in protection, but they do reduce the severity of clinical illness should it occur.
More recently, a live, attenuated S. equi vaccine (PinnacleTM I.N. by Fort Dodge) has been introduced as an intranasal vaccine for the prevention of strangles. The vaccine is administered twice, at an interval of 1–2 weeks. This approach to vaccination is intuitively more attractive than a killed, intramuscular vaccine since it produces the local antibodies necessary for protective immunity. Because the vaccine is a live but attenuated (using a low virulence organism) S. equi, take care to avoid contamination of injections elsewhere in the horse. Concurrent injection of other vaccines has resulted in S. equi abscesses at these sites, presumably through inadvertent contamination. Therefore, it is strongly recommended to not administer other vaccines or injections at the same time as administering the intranasal vaccine — or to be very careful about preventing contamination of injection sites. Other adverse reactions have also been reported. According to the manufacturer, adverse reactions occur at a frequency of about 5 per 10,000 doses. These include submandibular and pharyngeal lymph node swellings, with or without abscessation, purpura haemorrhagica, which may be severe, and even bastard strangles. Since the live organism may persist in the nose, approaches to control that involve detection of carriers may not be effective in horses immunized with this vaccine.
Immunity
After developing strangles, most horses eliminate infection fairly rapidly (i.e., within 30 days after recovery). Approximately 75% of horses develop a solid enduring immunity to strangles following recovery from the disease. (2) However, individual animals may remain with infection persistent within the guttural pouch, and may secrete the organism in nasal exudate or saliva for months or years. These carriers show no evidence of clinical disease and are the major source of infection for other horses with which they are mixed.
Control of Strangles
Isolate clinically affected animals or identified carriers immediately in a quarantine area, and clean and disinfect their water buckets or feed containers daily. Bedding can be burned or alternatively composted under a plastic sheet (to prevent spread by flies). Scrub with water and detergent any areas contaminated by infected horses, then disinfect by steam cleaning and/or applying effective disinfectants. Fly control is required to prevent spread during an outbreak.
Under optimal conditions, the bacteria can survive probably 6–8 weeks in the environment. Jorm (1991) has shown that S. equi survived for 63 days on wood at 2°C and for 48 days on glass or wood at 20°C. (6) The organism is readily killed by heat (60°C) or disinfectants (particularly povidone iodine, chlorhexidine). Rest contaminated pasture areas for 4 weeks, since the natural antibacterial effects of drying and of ultraviolet light will kill the organism.
Have quarantine area staff change their coveralls and boots before leaving the quarantine area, and wash their arms and hands carefully with chlorhexidine soap.
Approaches used to control strangles will depend on the circumstances of the individual horse or horse farm, but all people involved with horses need to maintain constant vigilance. These approaches involve a combination of knowledge of the history of individual animals and their source of origin, general hygiene, quarantine, and immunization, with appropriate action if an outbreak occurs.
Farms with large populations and movement of horses, particularly of older foals and yearlings, will want to maintain a routine immunization program of all horses to reduce the incidence and severity of disease. On these farms, depending on the vaccination program including the type of vaccine used, all incoming horses should be isolated for 2 to 3 weeks and, although expensive, a series of nasal or preferably nasopharyngeal swabs taken during this time for demonstration of the organism or its DNA. Only then should these isolated horses join the rest of the group.
Where a few adult horses are kept together and are uncommonly mixed with other horses, immunization may not be required since all immunization carries a slight risk of adverse effects. Incoming animals should be quarantined for 3 weeks, during which time nasal swabs should be assessed for the presence of the organism.
Source: omafra.gov.on.ca



